

- Home
- ABOUT US
- PRACTICES
- QMS & REGULATORY SERVICES
- Design, DEVELOPMENT & TESTING
- MANUFACTURING
- CAREER
- Contact




We outsource biocompatibility testing as per ISO 10993 & according ISO 10993 matrix by certified labs.
We use only biocompatible material for each invasive device.
Our products are Non –Toxic & Non-Pyrogenic.

We perform risk based evaluation & hence manage to reduce the risk to the most possible levels. On the basis of risk based chart we identify the Hazard with respective severity/ consequence of Harm & their probability of reoccurrence.
Flow for Evaluation are as
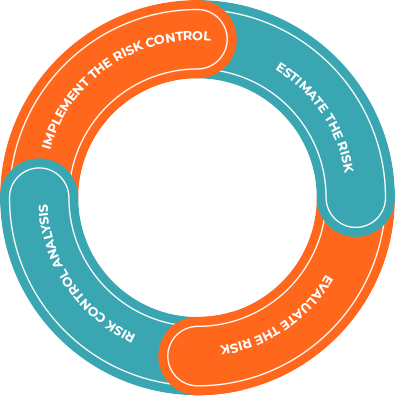
It is intended to have no risk remaining in the red region.
All remaining risk shall possibly be in the green region.
Risk remaining in the yellow region has to have a clear medical rational.
Risks which do not appear in the acceptable region after implementation of all risk control measures will always be communicated to and approved by the management.
Any Risk remaining in the S-5 region will be specifically observed by post-market surveillance and the first occurring event is going to trigger an escalation plan.
Check list of Medical Device Directive 93/42/EEC as amended by 2007/47/EC are followed for complying the essential requirement. Essential Requirement major criteria’s are as below
Design for intended use, Raw Material Selection, Storage & Preservation, Manufacturing Process & Control, Packaging, Labeling, Symbols, and Product Insert.
Testing of critical parameters. Bioburden, Biocompatibility, Sterility, Toxicity, Pyrogenicity, Stability/Shelf Life, Hygiene of Products, adequate Labeling, and adequate Information have been carried out ethically.

We perform the clinical evaluation in accordance with ISO 14155 & MEDDEV. 2.7.1 Rev.3;through the medical experts.
Literature review/Clinical Experience/Clinical Investigation
Comparison with Predicate Device
Customer complaints Review
After gathering the enough information we perform Clinical data analysis & implement necessary changes if required & made final conclusion.
As a supplementary to clinical evaluation we routinely perform the Post Market Clinical Follow Up.
A strong vigilance system helps us in performing the any reporting in very ethical way as per MEDDEV 2.12-1 rev 8 or as amended.
