

- Home
- ABOUT US
- PRACTICES
- QMS & REGULATORY SERVICES
- Design, DEVELOPMENT & TESTING
- MANUFACTURING
- CAREER
- Contact


We will gladly assist in the creation or improvement of your usability engineering procedure and facilitate the transfer of individual process steps, such as task analysis, risk analysis for application error, specification of the usability requirements, creation of validation plans, development-process usability tests, as well as verification and validation of the usability. In addition, we are glad to offer the creation of a standardized usability engineering file, including the closing report according to FDA requirements.
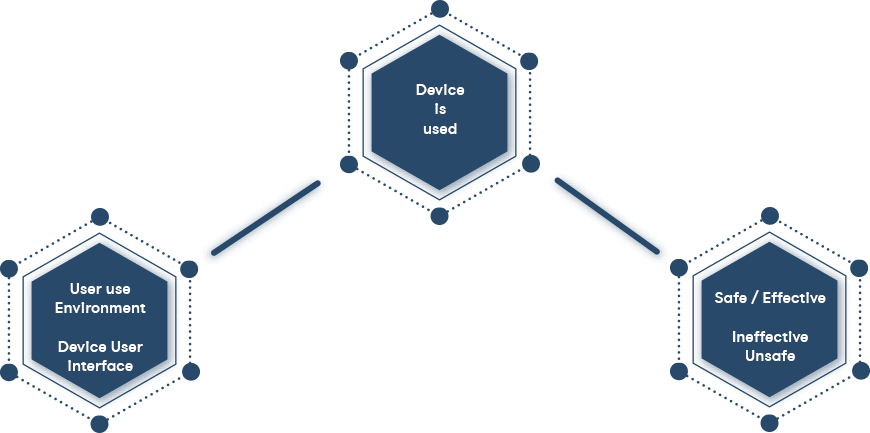
The aim of a usability engineering process is to deliver product of easy to use and safe in the intended context of use, and by intended users (whether by carers or patients themselves). Users should not have to read, understand and remember complex instructions for use and adapt to the requirements of the device, or use it in an uncomfortable, incorrect and possibly dangerous way: a well-designed product will be easy to use, and will have a user interface that is consistent with user experiences and expectations.
Use error includes the inability of the user to complete a task
Use errors can result from a mismatch between the characteristics of the user, user interface, task, or use environment
Users might be aware or unaware that a use error has occurred
An unexpected physiological response of the patient is not by itself considered use error
A malfunction of a medical device that causes an unexpected result is not considered a use error.
There can be more than one user of a medical device
Common users include clinicians, patients, cleaners, maintenance and service personnel.
Accompanying documentation is considered part of the medical device and its user interface
User interface includes all the elements of the medical device with which the user interacts including the physical aspects of the medical device as well as visual, auditory, tactile displays and is not limited to a software interface.