
Components Moulding
Components moulding with hot run & cold run technology with screw or force ejection system.


Medzus Medical manufactures medical devices in strict compliance with International manufacturing and regulatory requirements, all products are produced and packaged within state-of-the-art clean room and controlled environments in compliance with EU medical directives and Good Manufacturing Practice (cGMP).
Production facilities have been constructed and maintained in accordance with the GMP requirements for cleanliness. Plant operations are maintained for high quality standards by qualified and experienced technical personnel and trained workforce. All employees are trained regularly for personal hygiene and clean room environment. Utmost attention is provided to minimize contamination from external sources.

Components moulding with hot run & cold run technology with screw or force ejection system.
Clear, Radiopaque & Multilumen extrusion with fully automatic advanced technology.
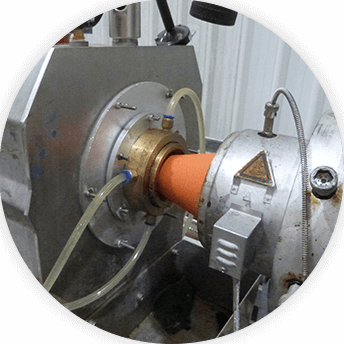
Product cutting (Mechanical or Laser Techniques)
Catheter tipping (Open tip type)
Forming (Close or Open type)
Hole punching (Mechanical or High Frequency)
Bonding (Adhesives or UV curing)
Welding (High Frequency & Ultrasonic)
Sutures swaging & crimping

Medical grade pouch packaging
Tyvek & medical grade blister packaging,
Aluminum blister moisture proof packaging.
Ethylene Oxide Gas Sterilization
Gamma Sterilization