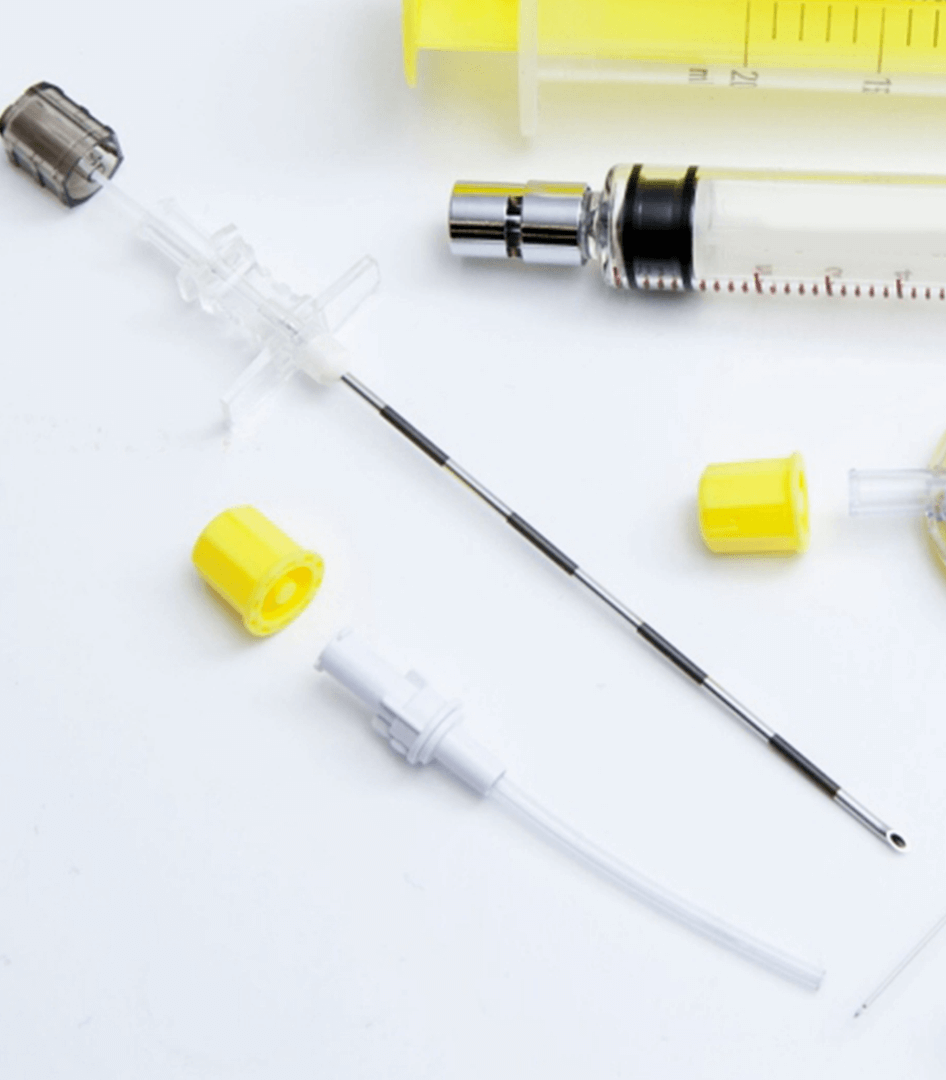
Neuraxial devices, such as epidural catheters, are used to deliver medicines or anesthesia to neuraxial sites, such as the epidural space, or are used to monitor or remove cerebral-spinal fluid for therapeutic or diagnostic purposes. ISO 0369-6:2016, was published in March 2016, to provide specifications for designing the connectors for use with neuraxial devices. Medzus Medical recognizes this standard.
Gauging Test
Dimensional Analysis
Falling Drop Positive Pressure Liquid Leakage
Sub atmospheric Pressure Air Leakage
Stress Cracking
Resistance to Separation from Axial Load
Resistance to Separation from Unscrewing
Resistance to Overriding